Flex Lab Research Spotlights
Northwestern and its research partners are using the Flex Lab to forge new frontiers in the fields of sustainability and energy.
Sustainability at the Energy-Water Nexus
 Aaron Packman, Professor of Civil and Environmental Engineering and Director of Northwestern’s Center for Water Research, is utilizing the Flex Lab in a partnership with Argonne National Laboratory and several European research institutes. The project will develop new low-cost, low-energy water purification technologies and address the need for high-frequency, high-resolution sensors that will enhance current water monitoring protocols, providing improved data to policy makers and scientists.
Aaron Packman, Professor of Civil and Environmental Engineering and Director of Northwestern’s Center for Water Research, is utilizing the Flex Lab in a partnership with Argonne National Laboratory and several European research institutes. The project will develop new low-cost, low-energy water purification technologies and address the need for high-frequency, high-resolution sensors that will enhance current water monitoring protocols, providing improved data to policy makers and scientists.
Solar Fuels Institute (SOFI) Demo Project
Justin Notestein, Professor of Chemical and Biological Engineering, and graduate student Alex Grant are using the Flex Lab to experiment with new catalysts designed to capture ambient carbon dioxide and convert it into methanol. The small-scale reactor leverages solar energy to generate the electrical and thermal energy needed to drive water electrolysis for renewable hydrogen, direct air capture of carbon dioxide, and ultimately methanol production. The research is phase one of SOFI’s four-phase project that involves developing a safe, economically viable system to convert carbon waste into energy dense fuels.
Solid Acid Fuel Cell Stack for Distributed Generation Applications

Sossina M. Haile, Professor of Materials Science and Engineering and Applied Physics, is using the Flex Lab to carry out her Advanced Research Projects Agency-Energy (ARPA-E) research grant from the U.S. Department of Energy. Her interdisciplinary project seeks to improve the operation of electrochemical devices and has implications for fuel cells, electrochemical ammonia generation cells, and hydrogen producing electrolysis cells. Specifically, her research will prepare nanostructured electrodes incorporating the electrolyte cesium dihydrogen phosphate (CsH2PO4).
Building Catalysts for a Hydrogen Economy
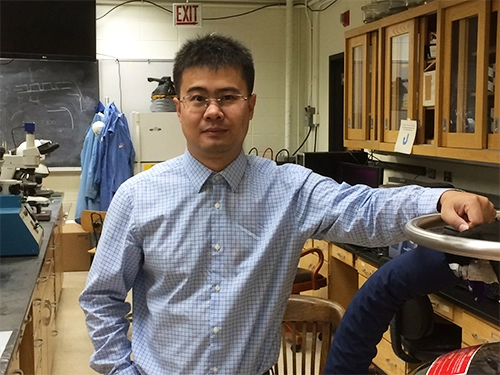 Utilizing hydrogen in place of fossil fuels is a promising clean energy alternative. Designing catalysts that are able to generate hydrogen, convert it into consumer-friendly energy resources, and upgrade chemicals to meet the needs of industry is an important pillar of the hydrogen economy. In the Flex Lab, Visiting Professor of Chemistry Yijin Kang and his team are focused on understanding and improving the catalysts that are used in fuel cells, electrolyzers (e.g. water splitting), batteries, and chemical upgrading. Their approach includes: 1) understanding catalysis by well-defined model materials and computational tools, and 2) designing highly efficient catalysts by tailoring catalyst nano-structure and fine-tuning the useful composition (e.g. development of single atom catalysts).
Utilizing hydrogen in place of fossil fuels is a promising clean energy alternative. Designing catalysts that are able to generate hydrogen, convert it into consumer-friendly energy resources, and upgrade chemicals to meet the needs of industry is an important pillar of the hydrogen economy. In the Flex Lab, Visiting Professor of Chemistry Yijin Kang and his team are focused on understanding and improving the catalysts that are used in fuel cells, electrolyzers (e.g. water splitting), batteries, and chemical upgrading. Their approach includes: 1) understanding catalysis by well-defined model materials and computational tools, and 2) designing highly efficient catalysts by tailoring catalyst nano-structure and fine-tuning the useful composition (e.g. development of single atom catalysts).
Formaldehyde and Hydrogen Reactor for Bio-methanol Upgrading
 Tracy Lohr, Research Assistant Professor of Chemistry, is using the Flex Lab to experiment with a new process designed to convert bio-available methanol to formaldehyde (produced worldwide at 30 million metric tons/year) and hydrogen fuel. The bench top prototype reactor is designed to yield high single pass conversion to formaldehyde while leveraging co-produced hydrogen to fuel the process, thereby making the process energy neutral in contrast to commercial formaldehyde production. Her work will focus on prototype testing for high conversions and formaldehyde capture technology.
Tracy Lohr, Research Assistant Professor of Chemistry, is using the Flex Lab to experiment with a new process designed to convert bio-available methanol to formaldehyde (produced worldwide at 30 million metric tons/year) and hydrogen fuel. The bench top prototype reactor is designed to yield high single pass conversion to formaldehyde while leveraging co-produced hydrogen to fuel the process, thereby making the process energy neutral in contrast to commercial formaldehyde production. Her work will focus on prototype testing for high conversions and formaldehyde capture technology.
Studying the Fundamental Materials in Lithium-ion Batteries
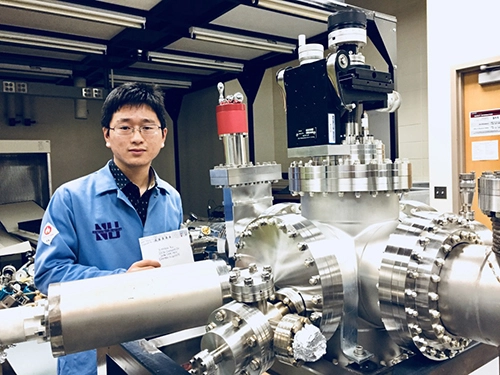 As part of the Center for Electrochemical Energy Science (CEES) at Argonne National Laboratory, Professor of Materials Science and Engineering Scott Barnett and graduate student Xiankai Yu are in the Flex Lab carrying out research on the interactions that occur at the interfaces between materials within lithium-ion (Li-ion) batteries. In particular, electrode materials such as LiMn2O4 can interact with the liquid electrolyte to form interfacial layers that may block Li-ion transport, and manganese (Mn) can be dissolved from the LiMn2O4, resulting in a loss in battery capacity over time. Barnett’s work focuses on making high-quality LiMn2O4 thin layers that are ideal for studies of interface reaction during battery operation. Additionally, he is carrying out fundamental studies of coating materials on Li-ion battery electrodes such as LiMn2O4, aimed at improving the cycle life of materials by eliminating Mn loss.
As part of the Center for Electrochemical Energy Science (CEES) at Argonne National Laboratory, Professor of Materials Science and Engineering Scott Barnett and graduate student Xiankai Yu are in the Flex Lab carrying out research on the interactions that occur at the interfaces between materials within lithium-ion (Li-ion) batteries. In particular, electrode materials such as LiMn2O4 can interact with the liquid electrolyte to form interfacial layers that may block Li-ion transport, and manganese (Mn) can be dissolved from the LiMn2O4, resulting in a loss in battery capacity over time. Barnett’s work focuses on making high-quality LiMn2O4 thin layers that are ideal for studies of interface reaction during battery operation. Additionally, he is carrying out fundamental studies of coating materials on Li-ion battery electrodes such as LiMn2O4, aimed at improving the cycle life of materials by eliminating Mn loss.
Pushing Solar Cells to the Limit
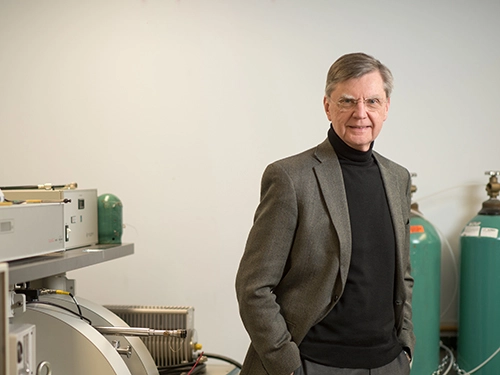 Through research conducted in the Flex Lab, Michael R. Wasielewski, the Clare Hamilton Hall Professor of Chemistry at Northwestern, is developing new materials to increase the capacity of solar cells by approximately 35 percent. As part of the Northwestern-Exelon Master Research Agreement, Wasielewski and his team are investigating an innovative application of a process called singlet fusion, which converts the energy in every photon of sunlight into two pairs of charges that are then collected by electrodes in the cell. Wasielewski and his team expect to deliver the new solar cell technology that can be fully commercialized.
Through research conducted in the Flex Lab, Michael R. Wasielewski, the Clare Hamilton Hall Professor of Chemistry at Northwestern, is developing new materials to increase the capacity of solar cells by approximately 35 percent. As part of the Northwestern-Exelon Master Research Agreement, Wasielewski and his team are investigating an innovative application of a process called singlet fusion, which converts the energy in every photon of sunlight into two pairs of charges that are then collected by electrodes in the cell. Wasielewski and his team expect to deliver the new solar cell technology that can be fully commercialized.
